AUBURN, WASHINGTON — In collaboration with Syntrix Pharmaceuticals, researchers at the University of Texas MD Anderson Cancer Center revealed that activation of CXCR2 in metastatic colorectal cancer (CRC) may explain why many patients do not respond to immune checkpoint blockade (ICB) therapy.
Findings from the study, published in the March 21 online issue of Cancer Cell, show that inhibition of CXCR2 by SX-682 increased sensitivity to ICB therapy. The study results newly reveal how KRAS, a key mutation common in colorectal cancer, acts to promote ICB resistance and metastasis by activating CXCR2.
CRC is a major cause of cancer mortality with about 1 in 5 patients presenting with metastatic disease at the time of diagnosis. Only about 1 in 10 patients with metastatic disease will survive to five years.
“The majority of colorectal cancer patients do not respond to immune checkpoint blockade therapy, motivating the need for study of mechanisms and combination regimens with targeted therapies and ICB,” said Ronald A. DePinho, MD, senior author of the study and professor of Cancer Biology at the MD Anderson Cancer Center.
Using a genetically engineered mouse model, DePinho’s team demonstrated how KRAS reduced expression of interferon regulatory factor 2 (IRF2). This in turn caused expression of CXCL3, a CXCR2 ligand. CXCL3 activation of CXCR2 on myeloid-derived suppressor cells (MDSCs) recruited them into the tumor microenvironment where they mediated immune suppression, metastasis and ICB resistance. The researchers found that therapeutic inhibition of CXCL3-CXCR2 signaling with SX-682 increased CRC sensitivity to ICB therapy and survival.
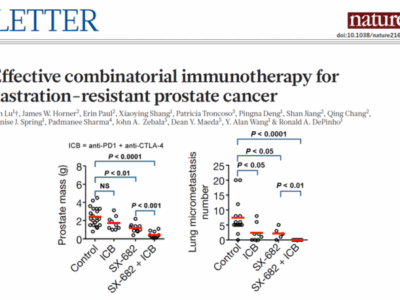
“Our studies suggest the use of combination CXCR2 inhibitor with ICB therapy in patients with advanced CRC who do not respond to today’s standard of care immunotherapy,” said DePinho. The study follows findings reported in Nature in 2017 by the same team that showed SX-682 made castrate-resistant prostate cancer “hot” to ICB and unable to metastasize.
ABOUT SX-682: SX-682 is a clinical-stage oral allosteric small-molecule inhibitor of CXCR1 and CXCR2 (CXCR1/2). CXCR1/2 are thought to be a combined “master switch” of the tumor immunosuppressive microenvironment (below).

Clinical studies have shown an inverse correlation between blood CXCR1/2 ligands and ICB response and survival. SX-682 has been validated in major solid tumor models, where it exhibits mono-agent activity, blocks metastasis, depletes MDSCs, activates infiltration and killing by immune effector cells, reverses chemo-resistance, and enhances ICB.
ABOUT SYNTRIX: Syntrix is a pharmaceutical company committed to discovering and delivering innovative therapies to solve the most difficult clinical problems. Convergent Science & Strategy. Breakthrough Medicines.
DISCLOSURE NOTICE: This release contains forward-looking information that is based on company management’s current beliefs and expectations and are subject to currently unknown information, risks and circumstances. Actual results may vary from what is projected. Syntrix does not undertake any obligation to publicly update these forward-looking statements, whether as a result of new information, future events or otherwise.
Media Contact: Aaron Schuler, PhD, 253-833-8009, x21
The company issued a press release.